Biofilm-fighting system for urinary catheters proves effective in simulated environment
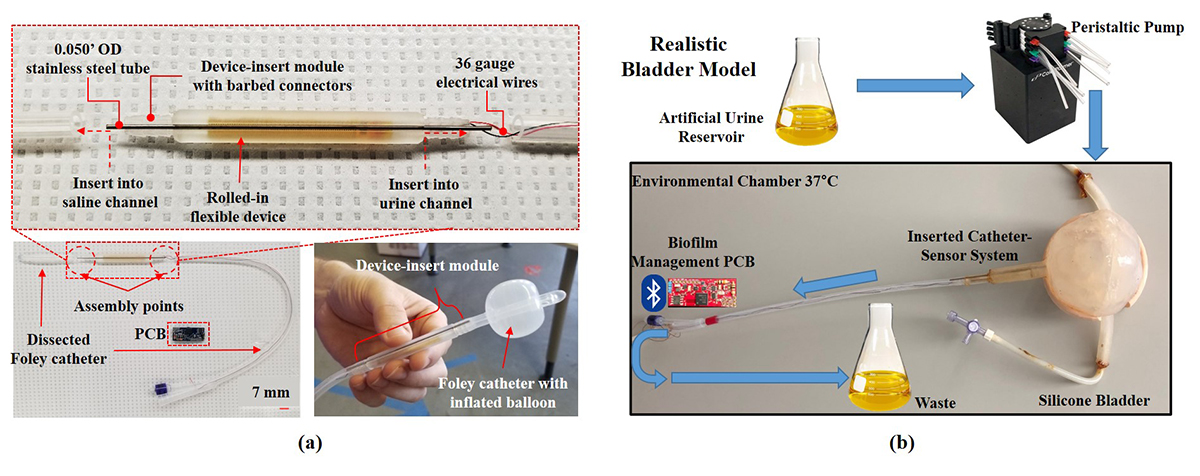
Fig. 1 from the paper shows a Foley catheter insert module and bladder model setup. (a) Components comprising the integrated system including steel tube for saline balloon inflation channel, 36 AWG wires to connect PCB with embedded sensor, flexible IDEs, and 3D-printed insert module with barbed connectors for leak proof connection with urine drainage channel. (inset) Optical image of insert module interfaced with 22 Fr Foley catheter with inflated balloon inflated. (b) Overview of realistic bladder model setup. Click on the image for a larger view. Bacterial biofilms are a major cause of infection in hospital settings and also are responsible for environmental biofouling. They are difficult to remove and contribute to the rapid increase in antibiotic-resistant bacterial strains. They can occur in an array of moist, inaccessible environments with complex curved geometries, such as urinary catheters, prosthetic implants, and water systems. In particular, catheter-associated urinary tract infections (CAUTIs), exacerbated by the emergence of antibiotic-resistant pathogens, are one of the most prevalent healthcare-acquired infections. Their presence often induces catastrophic consequences such as persistent infections, implant failure, systemic contamination, sepsis, and death. At the University of Maryland, work that originally began in 2007 continues apace on a flexible detection and treatment system that can be inserted in urinary catheters to detect, monitor and curtail the growth of biofilm. This integrated system uses the impedance of electricity to effectively monitor the biofilm, and treats it through the bioelectric effect, which combines low voltages of electricity with small dosages of antibiotics. In a new paper, “Integrated System for Bacterial Detection and Biofilm Treatment on Indwelling Urinary Catheters” this system is demonstrated in a commercial Foley urinary catheter and successfully tested in a newly-developed simulated bladder environment. The paper is forthcoming in IEEE Transactions on Biomedical Engineering and is available online via early access. The authors are alumnus Ryan Huiszoon (BioE Ph.D. 2020); former ISR postdoctoral researcher and alumnus Sangwook Chu (EE Ph.D. 2018); Electrical and Computer Engineering Ph.D. students Jinjing Han and Justin Stine; UMD Research Associate Luke Beardslee, M.D., Ph.D.; and Professor Reza Ghodssi (ECE/ISR). The integrated system is comprised of flexible, interdigitated electrodes incorporated with a urinary catheter via a 3D-printed insert for impedance sensing and bioelectric effect-based treatment. Each of its functions can be wirelessly controlled via Bluetooth from a user-friendly custom smartphone app. In tests, the system maintains the primary functions of indwelling catheters—urine drainage, balloon inflation—while it detects the growth of Escherichia coli bacteria with an average decrease in impedance of 13.0% after 24 hours. The system also is able to reduce the presence of biofilm through bioelectric effect-based treatment. This is performed by applying a low-intensity electric field that increases the susceptibility of biofilm bacteria to antimicrobials. The bioelectric treatment is projected to reduce the amount of antibiotics that need to be administered to combat the bacterial infection. This in turn will help to slow the further proliferation of antibiotic-resistant bacterial strains. This catheter system represents a significant step forward for a device-based approach to CAUTI management and could be adapted for use with other medical devices prone to bacterial infections, such as prosthetic implants. It also would be a valuable tool for scientists and clinicians working in biofilm research. In addition, the system could be modified for use in common moist and inaccessible environments that have complex curved geometries, such as water systems. Subsequent studies will focus on testing this system in vivo and in clinical settings. The system will soon incorporate additional sensors to target specific CAUTI biomarkers. The electrode device design can be modified to cover other vulnerable surfaces of the catheter. Finally, aspects such as safety and long-term stability will be explored. The work was funded by a TEDCO Maryland Innovation Initiative; the National Science Foundation; and the University of Maryland Medical Device Development Fund. An earlier version of this device won the University of Maryland’s 2018 Invention of the Year in the Life Sciences category for the “flexible urinary catheter insert to detect and prevent bacterial infections.”
Related Articles: April 2, 2021 Prev Next |