24 Teams Present at BIOE Capstone Competition Including First BCE Students
On Monday, May 8, the Fischell Department of Bioengineering (BIOE) Senior Capstone class presented 24 novel concepts at the annual Capstone Design Competition. Projects tackled a wide range of challenges and innovations, including an automated anastomosis device; machine learning to study proteins; dementia tracking using augmented reality; and a surgical tool to remove gastrointestinal polyps.
The Fischell Department of Bioengineering receives support for its Capstone Design competition from the Fischell Family, the University of Maryland MPowering the State Strategic Partnership, and industrial sponsor BD. Additionally, Capstone students often receive mentorship support from industry representatives and/or clinicians, including investigators from the University of Maryland School of Medicine, Children's National Health Center, the U.S. Food and Drug Administration, the University of Maryland Robert E. Fischell Institute for Biomedical Devices.
Team 1: PolypPal: A Novel Surgical Tool for Foreign Body Removal and Polyp Retrieval in GI Procedures Advisors: Dr. Guofeng Xie and Dr. Catherine Kuo FIRST PLACE Each year, there are about 28 million gastrointestinal endoscopies which involve removing foreign bodies such as polyps, blood clots, or foreign objects. Our device, PolypPal, is a novel surgical tool for foreign body retrieval to alleviate complications and enhance performance in gastrointestinal procedures. Due to the sheer amount of procedures completed, there is an increased need for more efficient retrieval devices to allow for more successful, uninterrupted operations. PolypPal’s mesh encloses around a foreign body to be retracted into an endoscope for removal. The current standard of care is unreliable with the retrieval mechanism and mesh breaking mid-procedure, which is costly and dangerous for patients under anesthesia. The PolypPal aims to eliminate procedure error, featuring a rotation and retraction mechanism for repeated use up to 5 times per procedure. The handling is sterilizable and reusable for attachment to a new mesh apparatus for other patients. Our prototype is tested for functionality in a simulated ultrasound gel-GI tract environment and during wire-mesh juncture durability tests. We hope to implement this prototype in the field with connections to the UMD Medical School and their researchers.
Team 2: Passive Noise Cancellation in a Hospital Environment Advisors: Dr. Giora Netzer, Dr. Helim Aranda-Espinoza, Dr. Nirupam Roy, and Mary Evans High hospital noise levels disrupt patient sleep, resulting in multiple health related issues. These include delirium and elevated fall risk. Additionally, sleep deprivation may cause immunosuppression and impaired wound healing, extending hospital stay. Therefore, there is an acute need to reduce hospital noise levels to facilitate better patient recovery. Current solutions for reducing hospital noise relying on changing hospital staff behavior are time-consuming and often ineffective. We sought to design a passive noise reduction device to reduce patient-perceived hospital noise levels. For cost-effectiveness and for immediate impact, this device uses a readily available hospital pillow as its direct medium for noise mitigation. The proposed device folds the hospital pillow over the patient’s ears and is designed to be easy-to-use for both patients and nurses and comfortable so as to not disrupt patient sleep. Furthermore, the device is designed to comply with hospital cleaning and sterilization protocols and may be continually reused. An initial prototype obtained a maximum decibel reduction of 4.4 decibels of noise levels sampledfrom the University of Maryland Medical Center. Additionally, surveying of patient and nursing stakeholders found that both groups would be willing to use such a device. The final prototype incorporates additional acoustic metamaterials formed from polyetherimide film on steel baseplate and latex noise reduction coatings. The device also features a more attractive, less bulky design, achieving greater noise level mitigation and higher comfort for patient use. This device will ultimately assist patients in noisy hospital environments, resulting in overall improvements for patient sleep quality and better overall recovery. Given the potential decrease in injuries and their resultant costs, the value proposition and market for this device is large.
Team 3: An Attachable Camera for Bedside Assessment of Commonly Placed Gastrointestinal Medical Devices Advisors: Dr. Katharina Maisel (UMD Department of Bioengineering), Dr. Guofeng Xie, Dr. Erik von Rosenvinge, and Dr. Andrew Leopold (UMD School of Medicine) THIRD PLACE Currently, the standard for hospital bedside gastrointestinal procedures typically involves “blind” insertion of medical devices through the nasogastric or orogastric routes. While this non-image-guided procedure is generally 60-80% successful, there can still be significant risks and discomfort to the patient associated with improper insertion into the airway instead of the GI tract. To help with the insertion process, we created a prototype of an adaptable, attachable camera for existing GI intraluminal tubes. The device consists of a camera, LEDs, and plastic housing, and can be retrofitted to existing GI catheters of varying sizes using a polyurethane adapter. We conducted several tests on the housing and camera, including resolution and brightness tests and thermal testing, and were able to collect data on resolution and brightness using a USAF 1951 1X resolution chip and nano LEDs. The results showed that the camera had a resolution of 1.782 lp/mm for the four LED setups with a pixel size of around 280.6 μm. We also found that the four LEDs produced 1660 lux, a light level comparable to industry-standard endoscopes. Thermal testing was also performed on the device, yielding a maximum temperature of 56 °C which is significantly lower than the current accepted maximal medical standard of 78.6 °C for gastrointestinal procedures. With this device, we hope to provide low cost visual support for these procedures, which will result in reduced patient discomfort, decreased adverse medical events and improved patient care while saving hospital resources.
Team 4: Preterm Infant Colorimetric Assay for Necrotizing Enterocolitis (PICNEC) Advisors: Dr. Rose Viscardi and Dr. William Bentley Necrotizing enterocolitis (NEC) is a gastrointestinal disease that affects around 7-10% of preterm infants and is often associated with a high mortality rate. The disease progresses rapidly with most deaths occurring within 24-48 hours after the initial onset of symptoms. Early clinical symptoms are difficult to distinguish from those of food intolerance, and current diagnostic tools are time-consuming and expensive, highlighting the need for rapid screening for NEC to facilitate quicker treatment implementation, prevention of NEC progression, and lower treatment cost. The objective is to develop a stool-based, rapid, and non-invasive colorimetric assay to screen for the risk of NEC in preterm infants by detecting the presence of the short-term fatty acid formate using potassium permanganate, a colorimetric indicator. In this project, the researchers develop a standard curve for clinically relevant formate concentrations, work with stool samples to quantitatively and qualitatively determine the risk levels, and develop a physical prototype for the device to account for ease of use and speed. While this project is proof of concept, with this device researchers will be able to improve the quality of life for preterm infants with NEC and reduce the likelihood of long-term complications such as intestinal failure, short bowel syndrome, and death.
Team 5: Measuring Fructose in Blood for Early Liver Cancer Detection Advisors: Dr. Sui-Seng Tee, Dr. Yang Tao, Dr. Maurizio Cattaneo, and Anjana Hevaganinge Liver cancer is one of the most prevalent and lethal types of cancer in the United States. It has a low survival rate and the imaging technologies used to detect liver cancer such as MRIs, CT scans, and angiograms are expensive, slow, invasive, and not available as point-of-care devices. However, hepatocellular carcinomas have been correlated with decreased levels of fructose metabolism and higher concentrations of fructose in the blood. Changes in fructose concentrations can be used as a marker for hepatic cancer. Our group’s project therefore involved the use of novel hyper-spectral imaging (HSI) techniques to detect fructose concentrations in various samples. Specifically, a HSI camera was used to image solutions consisting of either water or horse serum containing different ratios of fructose and glucose. The data was then used to train a neural network to model the correlation between the transmittance data from the HSI system and the fructose concentration that was imaged. We found that the neural network was able to effectively differentiate fructose from glucose. It also had an R2 value greater than 0.9 on an unseen validation dataset, indicating that it can successfully use HSI data to predict fructose concentrations in serum samples. In the future, this technology could be minimized in order to be used as a point-of-care device. An ethical impact of this research would be the ability to inexpensively screen for hepatic cancers in their early stages. This would avoid expensive treatments associated with late stages of liver cancer and be available to more people than current diagnostics.
Team 6: Single-Stage Percutaneous Tracheostomy Device Advisors: Dr. Joseph Rabin and Dr. Tao Lowe Percutaneous tracheostomies have become a common practice in intensive care units worldwide, with over 100,000 procedures performed annually in the United States alone (Yu, 2010). Currently there are health risks associated with the standard of care for tracheostomies, as 5.7% of tracheostomies result in bleeding. The current procedure for tracheostomies involve the use of multiple instruments and several step-wise operations that potentiate the risk for the patient to lose their airway. Risks for patients caused by current practices include bleeding, tracheal fracture, hypoxia, and asphyxiation. The team created a novel surgical tool to dilate the tracheal stoma and load the tracheostomy tube in a safer, more streamlined fashion. This new design utilizes soft, medical-grade plastic, metallic flanges, and a living hinge that allow for precise dilation at the site of the surgery. The device consists of a plunger that fits into a hollow shaft with 5 pointed flanges on the end. Initial prototyping and testing yielded promising results – using different materials such as Aligus 30 and TPU, Solidworks Simulation determined that our device could dilate to half the required diameter before breakage. Incorporating all of the stepwise processes of a tracheostomy into a single tool makes it such that the procedure can be performed in a single step very quickly. Because of this, our device has the potential to improve tracheostomy patient outcomes in hospitals globally.
Team 7: Sequential Compression and Cooling Device Abstract Advisors: Dr. Louis Marmon and Dr. Edward Eisenstein ADVISORY BOARD AWARD FOR TRANSLATIONAL DESIGN Hyperthermia is the state of having a body temperature above 40°C, and can result in complications such as brain swelling, muscle damage, and even death if left untreated. The severity of complications is primarily dependent on the duration of elevated body temperature, making rapid cooling of the body extremely important. The efficacy of traditional cooling methods, such as ice baths and cooling blankets, is limited by vasoconstriction, a physiological response where peripheral blood vessels tighten when exposed to cold, which decreases blood flow. In order to circumvent this issue, we incorporated sequential compression, a technique used in therapeutics to improve circulation in the limbs, into a cooling blanket. By simultaneously applying cold to the skin surface and squeezing the lower extremities, we force cooler blood towards the body core, resulting in an increased cooling rate. We were able to construct a prototype which effectively meets our objectives. Our device pumps a 90:10 ratio of water and isopropyl alcohol at near freezing temperatures into a blood pressure cuff which then squeezes and deflates at a user-defined interval. Furthermore, the prototype meets our weight and size requirements for use in a clinical setting and is user friendly. We believe that our device will have a positive impact on patients with hyperthermia by reducing patient discomfort during treatment and decreasing the length of their hospital stay.
Team 8: Reducing Nitric Oxide Trapping in Hemoglobin-Based Oxygen Carriers Advisors: Dr. Allan Doctor and Dr. Alisa Morss Clyne Severe hemorrhage is the leading cause of death for trauma patients, both in civilian and military sectors; the resulting focus on development of an artificial blood replacement has spanned decades. Previous attempts at designing hemoglobin-based oxygen carriers (HBOCs) demonstrated promising oxygen transport but exhibited notable safety issues due to unfavorable nitric oxide (NO) sequestration and subsequent vasoconstriction. Erythromer (EM) is a first-in-class synthetic blood substitute which utilizes lipid membrane-bound hemoglobin to transport and dispense oxygen at rates similar to organic red blood cells (RBCs), but its NO sequestration properties are still suboptimal. The lipid shell contains a minimal amount of polyethylene glycol lipids (PEG-DSPE) for improved systemic circulation, but can be redesigned to benefit the rate of NO sequestration. This report shows the results of several NO sequestration, vascular ring, and microfluidic assays, as well as computed flow dynamics renderings, to investigate the optimal membranous PEG design for improved NO uptake and flow properties. The capstone group achieved a reduction in rate of NO sequestration in a modified EM design containing 5% PEG-DSPE, and identified promising results when increasing the molecular weight of PEG chains. EM redesign is informed and supported by streamline characterization of EM nanoparticles with varying membrane properties, and the predicted impacts on biocompatibility are confirmed in ex vivo induced vasoconstriction of rabbit aorta. The results of this design project provide biocompatibility data on current EM and future HBOC modeling and development for use as a life-saving blood replacement in trauma settings.
Team 9: Tunable Metamaterial Helmet for Enhancing Low-Field MRI Advisors: Dr. Austin Tapp and Professor Kevin Aroo The Hyperfine Swoop is an FDA approved system used for magnetic resonance imaging (MRI). It is a portable point of care machine that images patients without requiring their transport. It allows physicians to provide patients the care they need while giving them a more comfortable experience. However, the Hyperfine Swoop system has a weak magnetic field, which results in lower resolution images compared to traditional MRI systems. Our goal is to improve the resolution of the images produced by Hyperfine Swoop without making adjustments to the system itself. Therefore, we are developing a tunable metamaterial helmet to be worn by patients while they are imaged with the Swoop system. The helmet is made out of 3D-printed plastics and copper alloy wire. The primary goal of this project is to increase the signal-to-noise ratio of Swoop’s images by amplifying its resonance frequency signal with the helmet. Further, the helmet’s tunable qualities support shifts between higher and lower resonances, increasing the attainable range for resonance frequency matching - the mechanism that improves the quality of images produced by MRI systems.
Team 10: Novel Application of fNIRS to Diagnose and Monitor Pneumonia in Infants Advisors: Dr. Amal Isaiah and Dr. Giuliano Scarcelli Pneumonia is one of the leading causes of death in children worldwide, contributing to approximately 14% of deaths in children under the age of 5 globally. Disease burden is primarily concentrated in resource-limited regions, with 99% of all pneumonia-related deaths occurring in lower and middle-income countries and 80% occurring outside of a hospital. This project aims to design a low-cost, accessible, and sensitive pneumonia screening tool for such regions. We propose to use functional near-infrared spectroscopy (fNIRS) as the basis of a device that measures blood oxygenation levels in the lung tissue locally to detect pneumonia-associated hypoxia. Modeling our device off of previous low-cost fNIRS devices, we designed a wearable, Arduino-based system that uses two near-infrared wavelengths to measure concentrations of oxygenated and deoxygenated hemoglobin in the lungs. To test our device, we also designed a phantom to mimic the absorption properties of human lung tissue. This phantom consists of varying concentrations of copper sulfate and nickel sulfate (to mimic different blood oxygenation levels) encapsulated in polyacrylamide gel. Phantom properties were validated with an existing fNIRS device, Artinis PortaLite, and we aim to compare results obtained with Artinis’ device with our own. Future work will include designing and testing our device with a more physiologically relevant phantom that will account for inherent absorption/scattering properties of lung tissue and the miniaturization of our device for use in pediatric patients. Our prototype is a first step towards a device we believe will help mitigate global inequities in pediatric pneumonia care by promoting early diagnosis and more effective treatment.
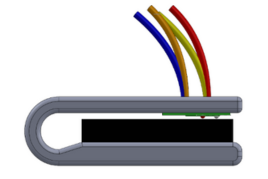
Team 11: Illuminated Laryngoscope Blade Advisors: Professor Brian Blair (BIOE), Dr. Louis Marmon (Children's National Hospital), and Dr. Marjorie Brennan (Children's National Hospital) BEST POSTER AWARD A laryngoscope is a tool used to either examine or insert a tube into the larynx – however, standard laryngoscopes have several major deficits. The blade has a side wall, known as the flange, that supports the light-propagating acrylic tube to the end of the laryngoscope. This flange reduces visibility by obstructing and offsetting the field of view. Additionally, it introduces an inherent right-hand bias to the device. The objective of this project is to improve visibility and eliminate the right-handed nature of laryngoscopes by removing the flange of the laryngoscope blade. Instead, the acrylic light propagation material will be built into the blade itself, so the flange is no longer needed. The novel acrylic blade will also be reinforced with aluminum and partially opacified via sanding to reach desired durability and light output ranges. This refigured laryngoscope would facilitate the diagnosis of upper airway medical conditions and make the placement of tubing during intubation procedures easier for clinicians.
Team 12: AAST Classifying Splenic Trauma using an Interactive GUI Advisors: Dr. David Dreizin and Dr. Kyung Koh Splenic trauma is one of the most common intra-abdominal injuries, with 40% of all abdominal trauma involving the spleen. While CT scans are the modality of choice to assess splenic trauma, injuries can be complex and difficult to interpret. Classification is therefore subjective, with modest inter-observer agreement. Subjective assessment prevents optimal management, which may be conservative, or include angioembolization or splenectomy. Suboptimal management results in increased morbidity and mortality. Round-the-clock trauma radiology expertise is limited to a few centers in the US. Frequently, trauma scans are interpreted after hours (when most severe trauma occurs) by trainees, or teleradiologists without trauma expertise. Explainable artificial intelligence offers a way to harmonize performance across levels of experience and subspecialization, while affording agency to the end-user in verifying, rejecting, or modifying results. Since AAST grading is a multi-step process, and the primary bottleneck of trauma CT scans is the report turnaround time (RTAT), AI CAD software with a concurrent reader capability can not only improve grading, and prediction of intermediate outcomes such as need for splenectomy, but can also decrease time effort, resulting in a faster report. A well-designed CAD tool can potentially reduce subjective measures of frustration and workload and improve user acceptance and likelihood of future use. There is currently no commercial CAD software for spleen trauma, and our prototype CAD tool addresses an important unmet need. Our solution to this unmet clinical need is currently an early technology readiness level mock-up. The mock-up combines our graphical user interface- evaluated favorably by expert trauma imagers- with previously designed back-end image processing for the primary features of AAST grading, with nnUnet for splenic laceration segmentation/quantification and Faster R-CNN for vascular injury detection. The tool is highly explainable and engendered a high degree of user trust. Further, in a two-reader cross-over clinical study with a junior and senior radiologist (n = 76), our final prototype demonstrated an approximate halving of interpretation time for splenic injuries, and improved clinical prediction for the junior reader (p < 0.05). Agreement (weighted k) between readers increased from between 0.40 and 0.59 without AI assistance to 0.72 with assistance. Furthermore, according to survey data, compared to other variations of our GUI, our final prototype displayed the highest likelihood of future use, clinician trust, perceived beneficence, mental support, diagnostic utility; additionally it yielded significant reductions in clinician effort, workload, and fatigue. Based on these findings, our GUI should have a significant clinical impact on assisting the training of new radiologists, and on aiding under-resourced clinics in their speed and confidence of classification of splenic injuries. It should accomplish this in an ethical manner without hindering the perceived beneficence of patient treatment.
Team 13: Developing a Point of Care (POC) Blood Test for Magnesium Advisors: Dr. Bhavani Kodali and Dr. Huang-Chiao Huang MPOWER AWARD Because magnesium helps maintain a steady heartbeat, healthy immune system, and bone strength, abnormal levels of magnesium have neurological, cardiovascular, or digestive implications that can result in death. In the past, the outdated method of colorimetric magnesium analysis has been used to examine magnesium levels. Now, our group is utilizing the technology of spectrophotometry for more rapid and accurate results. The aim of this project is to develop a more accessible and efficient method of diagnosing magnesium toxicity in those with magnesium-related disorders by making a point-of-care (POC) device that quickly measures magnesium with high sensitivity. In this method, a beam of light is passed through a sample solution of magnesium combined with an indicator reagent, calmagite, and the quantity of light the substance absorbs is measured. This is compared to a standard curve of magnesium and calmagite to determine the resulting magnesium concentration. Our ethical analysis concluded that consent, blood storage, and data storage will rely on HIPAA and hospital protocols to protect patients. The device itself is not able to retain data, as it is exported directly to a provider’s computer, making it difficult to violate patient rights. Our final prototype is a self-contained POC device designed to measure the amount of magnesium in a solution by averaging transmittance voltages over a time period of 10 seconds and converting it into concentration.
Team 14: The Characterization of Dementia Using HoloLens 2 Technology Advisors: Dr. Cha-Min Tang, Dr. Silvina Matysiak , Ian McDermott and Dr. Roger Eastman SECOND PLACE Dementia is one of the world’s fastest growing health issues. It involves cognitive decline in memory and other domains including personality, abstract thinking, and visuospatial skills. Dementia is of particular concern with the rise in life expectancy and is expected to triple in incidence by 2050.1 In 2023, dementia-associated costs in the US alone were estimated to surpass 345 billion dollars.2 The most widely used dementia screen—the Mini-Mental State Examination (MMSE) is not sensitive for mild impairment and is biased by the patient’s education level. More sensitive and unbiased diagnosis would pave the way for earlier treatment and improved quality of life.1 Previous literature has validated a positive association between MMSE scores and cognitive state as assessed by eye tracking of a region of interest.3 In order to create an alternative to the MMSE, a solution was developed in the form of an eye tracking simulation coded in Unity for the augmented reality headset HoloLens 2. This program evaluates the attention span of an individual by computing the radial error between eye position and a moving object projected in the augmented reality space. Prototyping and pilot testing of the HoloLens 2 program in the general population revealed that the program was able to differentiate between various cognitive states (e.g., alert versus tired) due to significant differences in radial error between the states. Future work will expand testing to dementia patients, where the results are expected to be more significant. Considering that dementia patients represent a particularly vulnerable subset of the population, the ethical impact of the study will be investigated while seeking approval from the Institutional Review Board. Additionally, as dementia patients have impaired cognitive abilities, the test execution will be as simple and user-friendly as possible to not pose an onerous mental burden.
Team 15: Neonatal Abnormal Movement Detection Advisors: Dr. Li-Qun Zhang (BIOE) and Dr. Paola Pergami (Children’s National Hospital and Georgetown University) Hospital The current clinical monitoring technique for neonates in the NICU involves constant observation, which can lead to fatigue and strain, resulting in over, under, or misdiagnosis of abnormal movements. Misdiagnosis of these movements can have severe consequences, including cognitive and behavioral comorbidities, higher mortality rates, and increased drug resistance and/or toxicity. In this study, we propose a solution to this problem in the form of a machine learning model paired with a graphical user interface to detect and display abnormal movements in neonates. The machine learning model consists of a Convolutional Long-Short Term Memory autocoder (CLSTM) trained to recognize anomalous activity in video data. The model uses anomaly detection strategies to distinguish between seizure activity and normal, healthy neonate movements in surveillance video. Currently, our team has created a working model structure, evaluation code, and a preliminary GUI design. The model can predict if a 6.7 second video clip contains anomalous activity with success rates far above our target 60%. After more evaluation, our team predicts that the model can perform significantly better than this baseline. Our solution minimizes the need for constant clinical monitoring, allowing healthcare professionals to have free hands and reducing strain while enabling accurate and timely diagnosis. Our approach has the potential to improve outcomes for neonates in the NICU, and future studies are needed to validate its effectiveness.
Team 16: An Improved Automated Anastomosis Device for Heart and Vascular Access Heart failure is one of the leading causes of death in the United States. Heart failure by itself affects 5.7 million patients in the US, and contributes to nearly 280,000 deaths annually. Patients that have been diagnosed with advanced heart failure often require mechanically assisted circulation, as heart transplantation is limited by the supply of donor organs. Mechanically assisted circulation is often achieved by using a left ventricular assist device (LVAD) for the heart. The main issue in using the LVAD is that it requires invasive surgical procedures to connect the device tubing to the aorta via suturing. This suturing process requires clamping of the aorta and placing the patient on bypass for an hour, so that there is no excessive blood flow during the surgery. This leaves room for physician error and blood leakage, which increases the patient’s risk for stroke. Our project involved the design of a specialized graft to allow for proper anastomosis without suturing, which would idealistically solve these issues. Our graft would simply be loaded into a sheath and deployed within a few seconds. The ability to insert the graft swiftly also eliminates the need for clamping the aorta, decreasing the risk of permanent damage or tissue injury. In terms of our design, nitinol wire’s heat-memory properties made it an ideal tool in the construction of our graph. We tested several different types with various activation temperatures, and found that the 30 degree wire performed best in a simulated biological environment. Deployment testing showed that our graft would reduce the time on bypass by nearly 25 percent, and the finalized prototype was able to withstand nearly the same amount of force, within 1lb, as the gold standard suturing method.
Team 17: DASH Robot Auto-Teach Strategy & Implementation Advisors: Dr. Tong Zhou and Dr. Yang Tao Blood culture is a technique used to detect fungi and bacteria in the blood and can be used as a good indication of patient blood infection. The Becton Dickinson DASH robot is a device intended to automate the process of culturing blood; the system consists of four rotating drums for incubation of the blood culture bottles and one robotic arm to retrieve and place bottles. Once fully functional, the robot will be able to grab a filled blood culture bottle, place it in a specific incubator slot, and retrieve it when necessary. While the DASH robot is fully functional, the robotic arm’s ability to consistently and precisely insert and remove blood culture bottles is flawed due to manufacturing limitations. During manufacturing, the robotic incubators have a tendency to come out imperfect, causing issues with bottle placement due to the misalignment between the robotic arm and incubator. With our group’s imaging system prototype, these manufacturing errors are accounted for by determining and correcting any misalignment. Once misalignment is detected and calculated with our imaging system, the robotic imaging interface will allow for the correct placement of any BD BACTEC bottles into the DASH system. Successful completion of this project entails an executed proof of concept design that will be implemented at Becton Dickinson and allow for the company to use imaging as a viable correction system. Overall, our project will allow for a smoother, automated process for blood culturing without the worry of incorrect bottle placement and offers the prospect of using this visual technology in other BD machinery.
Team 18: Solving Pulse Oximeter Racial Bias with Buccal Device Adivors: Dr. Jeffrey Hasday (University of Maryland School of Medicine, Baltimore) and Dr. Ian White (BIOE) Pulse oximeters are an accurate and non-invasive method for monitoring blood oxygen saturation (SpO2). More importantly, this method is widely used at home for those with respiratory impairments and in medical facilities for ongoing care. However, traditional fingertip blood pulse oximeters overestimate blood oxygenation for darker-skin-toned patients. To address this, we have developed a non-invasive buccal pulse oximeter to monitor oxygen saturation levels more accurately in all skin pigmentations. This device offers long and short-term measurements that emphasizes comfortability and size universality for different stakeholders. Based on a nylon thermoplastic encasing, this design holds an Arduino-based MAX30100 pulse oximeter sensor with a user-friendly OLED display for heart rate and SpO2 measurements. To ensure that the entire pulse oximeter remains sanitary, we are repurposing a plastic dental sleeve to surround the clip. Initial simulation results displayed our device's adaptability to various facial structures. Our project rectifies health outcome disparities that occur with the currently available pulse oximeter devices.
Team 19: Developing an Inhalable Dry Powder Nitrite Therapy Advisors: Dr. Jason Rose, Dr. Christopher Jewell, and Dr. Jenna Mueller STUDENT CHOICE AWARD Halogen gas poisoning is known to systemically lead to pulmonary injury. Chemical warfare, terrorism, and even industrial accidents can suddenly inflict mass suffering of innocent people in the way of airway constriction, hemoptysis (coughing up of blood), functional impairment, and a range of post-exposure effects including pulmonary hypertension (PH) that reduce quality of life. Outside of catastrophic events, PH is highly prevalent in people who experience preserved ejection fraction after diastolic heart failure (HFpEF), a condition with no cure or specific treatments. Patients with PH after HFpEF are subject to discomfort and numerous comorbidities due to irregular blood flow and oxygen distribution. Our team is proposing an inhalable dry-powder preparation of nitrite as a therapeutic treatment to reduce the post-exposure effects of halogen gas poisoning and for improving the function of the right-heart in patients with PH after HFpEF. We are partnering with Globin Solutions and expanding on their patented use of inhalable nitrite. Our method details encapsulation of a nitrite-excipient formula in a lipid nanoparticle (LNP) to prevent agglomeration of the highly hygroscopic small molecule in order to drastically increase shelf-life, particle stability, and overall efficacy of nitrite as a therapeutic. We plan to integrate our novel formulation into the existing dry powder inhalant market. After initial formulation via lyophilization, particle size distribution was inconclusive due to the hygroscopic nature of sodium nitrate. Particle size was evaluated via a Partica employing dynamic light scattering, a wet microscopy technique. When the sample was introduced into the Partica, the particles dissociated and dissolved, both freeing the ions and destroying the particle. In response, we pursued a lipid encapsulation method, both removing the potential clumping and allowing for proper testing. Our nitrite therapy has both broad and narrow implications for the future of pulmonary complications. With a shelf-stable treatment for gas poisoning that can be stockpiled, emergency deployment of our therapeutic could save both lives and money. The benefits of a medicine that can drastically improve the quality of life of victims of inflicted injuries can remedy the injustices of terrorism and industrial negligence.
Team 20: Applying Additive Manufacturing for Improved Purification of Extracellular Vesicles Advisor: Dr. Steven Jay Extracellular Vesicles (EVs) are small, membrane-bound capsules ranging in size from 40-100 nm that can be harvested from all body cells. They present promising new possibilities in the field of drug delivery and therapeutics for an array of diseases. EVs are at the center of innovative research regarding vaccine delivery techniques, but are difficult to purify due to their complexity and small size. The main challenge arises with the biomanufacturing process regarding EVs employment in bioengineering applications. Specifically, large scale production, integration, and continuous purification of EVs in downstream manufacturing. In this research project, our team utilizes additive manufacturing techniques such as 3D printing and computational fluid dynamic modeling, in order to design, create, and produce a spiral microfluidic retention device made specifically for EVs that allows for a continual purification. This device is based on the core principle of inertial sorting and size-exclusion chromatography (SEC). The device will then be tested using cell culture samples that include EVs in order to validate our device’s effectiveness at purifying the vesicles from the rest of the particles. This spiral microfluidic purification device for EVs exhibits promising new potentials for the manufacturing and industrial advancements of EVs to be utilized in the field of bioengineering.
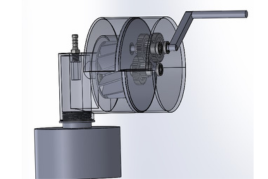
Team 21: Highly Portable Thoracic Drainage System for Military Medical Point of Care Advisors: Dr. Huckleberry Finne and Dr. Lex Schultheis Tension pneumothorax, the second leading cause of preventable death in combat situations, is an extremely life threatening condition characterized by the buildup of air or fluid in the pleural cavity and loss of negative pressure in the intrapleural space of the chest. In combat situations, this often occurs as a result of penetrating injury or blunt trauma to the chest, causing bleeding into the airway. On the battlefield, access to full hospital care is not always available, and existing emergency suction devices are not always practical due to reliance on batteries, large size, heavy weight, etc. This, in addition to the concerns that are radically unique to combat situations like performing emergency care under hostile fire, gives rise to the need for a practical emergency suction device for military medical personnel. The purpose of this project is to develop a portable, hand-powered, point of care suction device to enable chest trauma patients to receive sufficient care in combat casualty situations outside of a hospitalization setting. Improving upon the Triple Action Suction Thorax Evacuator (TASTE) prototype from researchers from the Uniformed Services University, we identified several aspects of the design that inhibited effective performance. Through several 3D printing iterations, we developed an improved model that both fits easily within a military medic’s rucksack and also provides effective suction. Validation of our final design includes the same bench testing as performed on the initial TASTE prototype and additional simulated-use experiments involving an anatomically relevant skeletal model to measure the pressure maintained and the suction rate. This data can then be directly compared to both the original TASTE prototype, and its major competitors, such as the NAR Tactical Suction Device and the V-VACTM Manual Suction Unit, to most effectively assess the success of our design. Through the development of a successful drainage device, we can improve accessibility and ease of operation for military medical professionals in the field. A successful device will not only allow combat casualties to receive the proper care they require, but may also help prevent deaths attributed to tension pneumothorax.
Team 22: Volumetric Blood Metering Device in Partnership with Becton Dickinson Advisors: Frans Feijen (BD) and Dr. John Fisher (BIOE) The primary focus of our project is to further explore two blood metering device concepts down selected by BD: 1) Paddlewheel Concept and 2) Pressure Sensor Concept. The overall goal of the blood metering device is to automate the blood collection process for improved sepsis diagnosis. Current collection methods rely on the pressure differential between the vacuum pressure in the collection bottle and the patient’s venous pressure to extract blood from vein to bottle. Blood collection is then manually terminated by the phlebotomist when the blood reaches the target volume line marked on the collection bottle; the manual nature of this process results in common cases of bottle under and overfilling. Considering sepsis diagnosis depends on the detection of microorganisms in blood culture, precise blood volume collection is critical for the patient to receive an accurate diagnosis. The blood metering device aims to solve this problem by automatically halting blood collection within the target volume range of 8-10 ml, removing the possibility of human error and increasing the sensitivity of sepsis diagnosis. BD’s proposed concepts include using a mechanical paddlewheel to translate rotations per minute into flow rate or using a pressure sensor that measures the pressure within the blood collection bottle and cuts off flow when the pressure reaches a threshold value. Our team explored both concepts and were able to successfully improve the accuracy of BD’s current paddlewheel prototype, develop a computational model that simulates blood draw events to inform future design, and propose a CAD design for the pressure sensor concept that is supported by simulated and experimentally collected pressure data. Our project outcomes will assist BD in determining the final concept for the device and facilitate future device development.
Team 23: Sequential Assignment of Protein and NMR Spectra by Computer Vision and Machine Learning Methods Recent research has shown that understanding protein structure and function can aid drug discovery efforts and enable the design of novel proteins with specific properties for various applications. Nuclear magnetic resonance has become the preeminent technique to study proteins and their dynamic interactions. The traditional methods of characterizing protein structures through NMR spectroscopy has led to a bottleneck in the drug discovery pipeline and has hindered progress in understanding protein function and interaction. Our proposed prototype aims to accelerate the process using computer vision and machine learning techniques. The prototype is a neural network that analyzes spectral graphics of unassigned amino acids, determining whether they are adjacent to each other in the protein sequence and where they are located in the overall protein sequence. In order to test the performance of our prototype, a combinatorial optimization technique was used to maximize the comparison score from the relative and absolute position. Therefore, the score of a successful model would outperform the score of the manual sequential alignment. Once trained, the proposed prototype could significantly benefit researchers and scientists in this field by automating the process of assigning NMR spectra, enabling faster and more accurate characterization of protein structures. This, in turn, could lead to the discovery of new therapeutic targets and the development of novel proteins for various applications. However, it is important to note that the impact of this technology may not be evenly distributed across the scientific community. Researchers who have access to the necessary computational resources and expertise may be more likely to benefit from this technology, while those who do not may be left behind. Additionally, the potential for bias in automated sequential assignment neural networks must be considered, as it could lead to discriminatory or misidentified outcomes. By taking a proactive approach to addressing these issues, we can ensure that the benefits of this technology are maximized while minimizing potential negative impacts. The use of machine learning and artificial intelligence in scientific research has the potential to transform the way research is conducted, enabling new discoveries and breakthroughs that were previously impossible. This could have far-reaching implications for fields beyond structural biology and drug discovery, including materials science, engineering, and environmental science.
Team 24: Particle Characterization using Fluidic Imaging Advisors: Professor Lan Ma (BCE) and Rigoberto Roche (Beckman Coulter) The classification of white blood cells is a significant topic of research in the field of hematology. Being able to accurately determine the concentrations of different types of white blood cells in a sample provides medical professionals valuable insight into possible diseases and risk factors. A number of artificial intelligence approaches designed to automate the classification process have been proposed in literature. However, all of these approaches are designed to work with microscopy images. Microscopy images distort the physical dimensions of the cells, casting doubt on how accurately they represent cells in vivo. There is a need for an automated blood cell classification tool that works on fluidic imaging data. This project proposes a method for automatic white blood cell classification designed for use on real-time fluidics images acquired from samples in solution. A novel convolutional neural network architecture was designed in TensorFlow. The model architecture was expanded based on a minimal working model. A number of regularization methods were implemented to improve the robustness of the network. The network was trained on a set of ~40,000 sample images per class. Input data was augmented to be robust against rotational and translational variance. The neural network was deployed in an Oracle Virtual Machine and evaluated for accuracy and speed. The model produced an overall classification accuracy of ~90%. Faster and more accurate classification of white blood cells can aid in the early detection and treatment of diseases, such as infections, leukemia, and autoimmune disorders. Research and development in this field can also advance our understanding of the immune system and contribute to the development of new therapies and treatments.
Related Articles: May 17, 2023 Prev Next |