Maryland Scientists Synthesize Metallic Glass Nanoparticles via High Temperature Thermal Shock
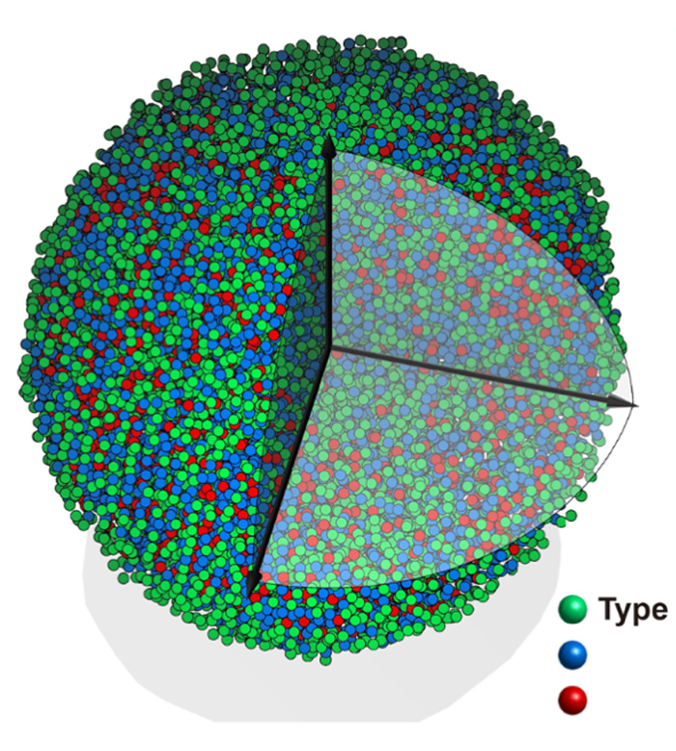
Amorphous materials are ubiquitous in our daily life and have found broad applications ranging from window glass and solar cells to telecommunications. However, synthesizing metallic glass nanostructures is a daunting task due to large differences among multiple glass forming elements as well as a lack of effective methods to induce glass formation at nanoscale, which typically requires a rapid quenching rate > 10^3 K/s. To make this process more efficient, a research team in the University of Maryland (UMD) Department of Materials Science and Engineering (MSE) for the first time synthesized a multinary metallic nanostructure dispersed on thin graphene substrates. This is achieved by using a unique far-from-equilibrium synthesis technique called 'high temperature thermal shock,' invented at UMD. Upon rapidly heating to a high temperature, these elements are mixed uniformly; rapid quenching (on the order of 10^5 K/s) immediately following could lead to rapid solidification, which is fast enough to avoid crystallization, thus forming the unique metallic glass nanostructures. The resulting metallic glass nanostructure was characterized at the University of California, Los Angeles (UCLA) in professor John Miao’s group where they discovered many unique features such as short-range order and crystal-like medium range ordering (image 1). These observations provide direct experimental evidence to support the general framework of the efficient cluster packing model in the glassy material community. This research was conducted through multi-disciplinary collaboration with research teams at UMD, UCLA and Berkeley National Laboratory. This study was published in Nature on March 31 (DOI: 10.1038/s41586-021-03354-0).
Related Articles: March 31, 2021 Prev Next |