UMD Research Group creates superior water-based battery chemistry
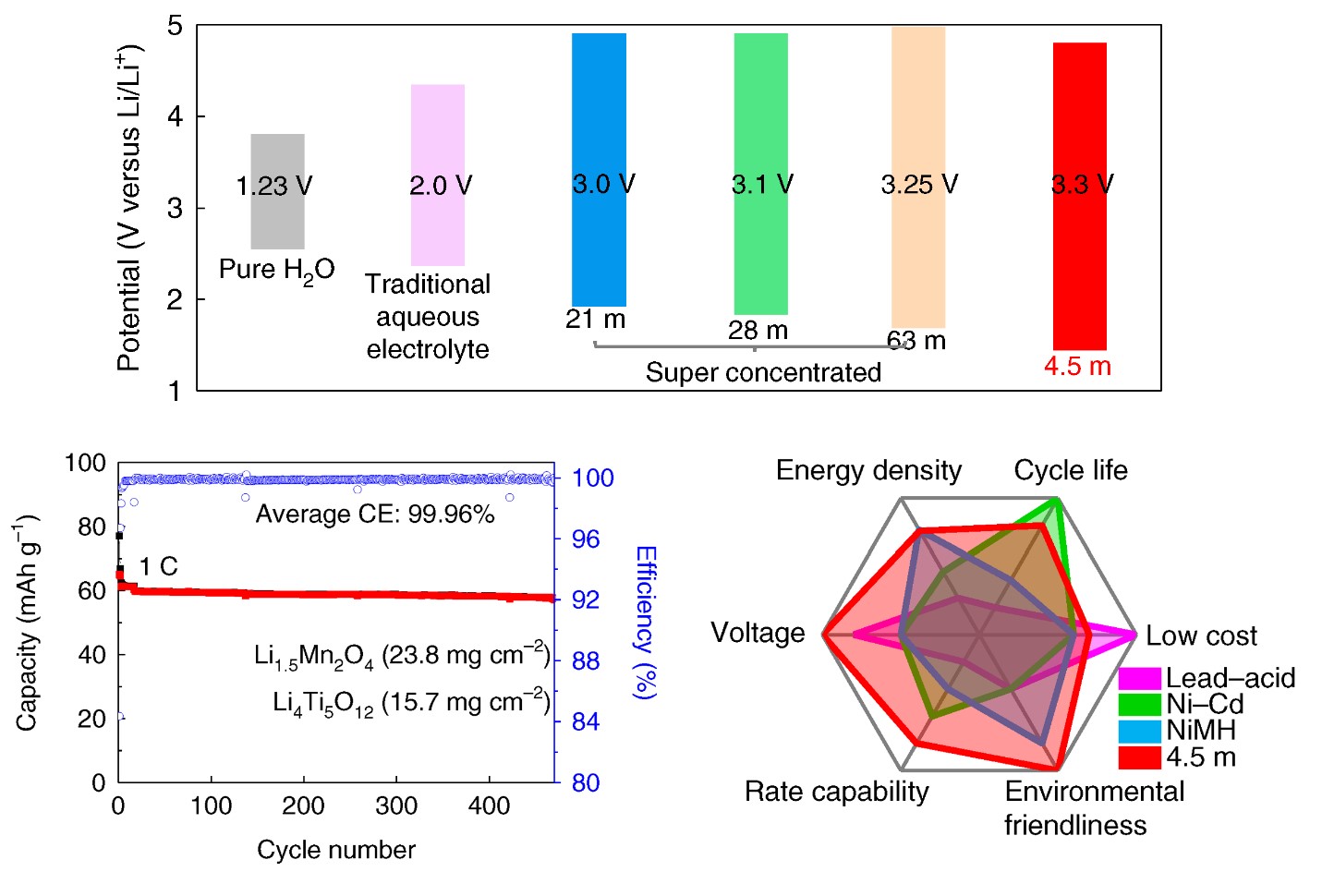
Looking to design next-gen aqueous electrolytes, beyond water in salt (WIS), while enhancing efficiency, stability and reducing cost, aqueous electrolytes with a lower salt concentration of 5.0 m (mol/kg) and wider electrochemical stability window of > 3.0 V are highly needed – and a research team at the University of Maryland (UMD) can provide that. The team, led by Chunsheng Wang – a professor in the UMD Department of Chemical and Biomolecular Engineering (ChBE) – reports in Nature Energy a non-flammable, ternary eutectic (e.g., mixing of three components) electrolyte that fulfills all the requirements. Jijian Xu, a ChBE Postdoctoral Researcher, served as the first author of the study. “We developed a next-generation aqueous electrolyte by reducing the salt concentration from 21m in ‘water-in-salt’ to 4.5m, while further extending the electrochemical stability window to 3.3V,” said Wang. “This electrolyte enables a 2.5V LiMn2O4 || Li4Ti5O12 pouch cell with practical settings, which is a big step towards commercial applications.” By mixing three eutectic components – LiTFSI, CO(NH2)2, and H2O, together with KOH as an additive, in this case – the team designed a robust, 4.5m water-based electrolyte, which expanded the electrochemical stability window to 3.3V with the cathodic limiting potential to 1.5 V. “This electrolyte can reduce the number of H2O in a Li+ solvation shell to 0.7 – the reduction of LiTFSI and CO(NH2)2 under KOH catalyst formed a robust LiF/polymer bilayer SEI,” said Xu. “Moreover, Li-rich Li1.5Mn2O4 is used as a lithium reservoir, which compensates for the Li-loss in the anode. Ultimately, we can achieve stable cycling with high Coulombic efficiency.” This battery chemistry – in addition to superior performance – is safe/non-flammable, low cost, and environmentally friendly, thus paving the way for the use of aqueous lithium-ion batteries in everyday applications. AquaLith Advanced Materials, a Maryland-based materials company, has licensed water-in-salt electrolyte battery technology for commercialization. The U.S. Department of Energy (DOE) has also invested $5M to development of water-in-salt electrolyte batteries. For additional information:
February 7, 2022 Prev Next |