UMD Researchers Design ‘Open’ Lithium-ion Battery
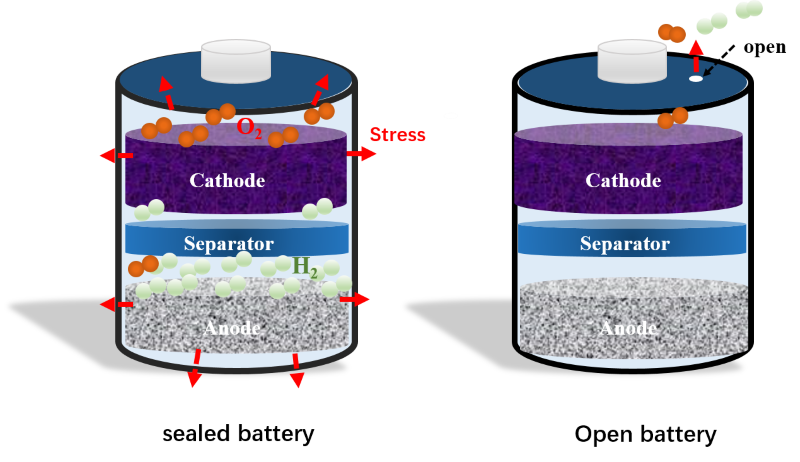
Lithium-ion batteries remain in high demand these days, which is why researchers continue putting extensive effort into design improvements. One critical challenge for non-aqueous batteries is related safety issues stemming from thermal runaway, a situation in which the temperature of a battery continues to increase, often resulting in damage. Although aqueous – or water-based – batteries use non-flammable, water-based electrolytes, they’re not immune to explosion. Overcharging, external short-circuiting, or crushing – in shipping transit, for example – can all lead to disaster. To solve this problem, a University of Maryland (UMD) research team led by professor Chunsheng Wang have not only reconfigured the Li-ion battery chemistry, but they’ve designed a new shell – this ‘open’ battery design boasting a water-based electrolyte is extremely safe, cost-effective and energy efficient. Long Chen, an assistant research scientist, served as first author on the study, published May 26, 2020 in Nature Communications. In a typical battery design, the shell is perfectly sealed – a flaw which can result in the buildup of pressure due to thermal runaway and gas evolution as the electrolyte begins to break down. If the gas has no place to go, the pressure builds, leading to explosion. “We designed an ‘open’ battery by placing a hole at the top of the shell, which allows heat and pressure to dissipate,” Chen said. “However, this open design could lead to oxygen from the air dissolving into the electrolyte, and oxidizing the discharged anode, thus destroying the battery, so we had to find a way to circumvent this possibility.” The key to this chemistry is salt – lots of it. “Using a high salt concentration for the water-in-salt electrolyte (WiSE), we were able to reduce the oxygen solubility and oxygen reduction reaction (ORR) kinetics,” Wang said. “A high salt concentration can also prevent water evaporation, thus preventing the battery’s electrolyte from running dry. These characteristics can help the open, water-based Li-ion batteries operate stably.” The WiSE significantly expanded the electrochemical stability window of the aqueous electrolytes from 1.5 V to >3.0 V. Moreover, the cycling performance was gauged at over 1000 times the typical level. These open WiSE Li-ion batteries will have applications in hand-held and household electronics, automobiles, and beyond. For additional information: Chen, L., Cao, L., Ji, X. et al. (26 May 2020). Enabling safe aqueous lithium ion open batteries by suppressing oxygen reduction reaction. Nature Communications 11, 2638. https://doi.org/10.1038/s41467-020-16460-w
Related Articles: May 27, 2020 Prev Next |